Salinity Gradient Energy: Current Membrane Development and Challenges for Reverse Electrodialysis System
© 2016 by the New & Renewable Energy
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Salinity gradient power generation has huge potential to generate clean energy from mixing water streams of different salinities. Reverse electrodialysis (RED) is an emerging membrane-based energy conversion technique that drives such energy sources to electricity. In RED, the transport of ions in selective ion exchange membranes (IEMs) is employed for power generation. As a key component in RED power generation, the development and optimization of IEM are crucial for sustainable energy conversion from salinity gradients. Recently, the demand for high quality membranes that are designed uniquely for superb power performance is increasing. This review evaluated commercially available IEMs, currently available state-of-the-art RED membranes, and their key physical and electrochemical properties (e.g., resistance, permselectivity, and swelling). The crucial role of the membrane properties in salinity gradient power generation and their interconnected relationship are discussed through the RED system. This review highlights the importance of IEM development for effective energy harvesting from salinity gradients and provides a better understanding of the dominant factors in RED that directly determine the power performance. The use of proper membranes will expedite the wide-spread application of RED as the next promising renewable energy technology.
Keywords:
Renewable energy, Salinity gradient power, Reverse electrodialysis, Ion exchange membranes1. Introduction
With the need for a more energy secure world, further development of technologies that are both renewable and sustainable while being environmentally friendly is needed. The ocean is one such renewable energy source that has yet to be explored. Through the mixing of freshwater with ocean water, free energy is created, thus the chemical potential of river water and seawater convert into electrical energy. The amount of energy generated from the salinity gradient in the ocean, river water mixture has been shown to be equivalent to the energy generated from a ~270 m tall waterfall.[1~3] Salinity gradient energy is becoming more widely seen as a promising clean and sustainable energy source that is feasible due to the abundance of sea and river water. Reverse Electrodialysis (RED), which utilizes ion exchange membranes (IEMs), and pressured-retarded osmosis (PRO), a technology that uses semi-permeable membranes, are the two most feasible approaches to harnessing salinity gradient energy.
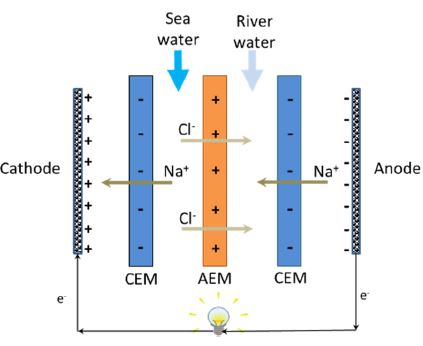
These two technologies are best used under different salinity conditions with PRO being more efficient in the mixing of concentrated brines, compared to RED, which is better used when mixing sea and river water.[4] The use of wastewater effluent with brines obtained from desalination plants has been looked at as another salinity gradient energy source but is limited by the need for better membrane and system optimization. PRO and RED meanwhile are often limited by the cost of their membranes. Also, the commercialization of PRO and RED is hampered by the performance deterioration of membranes. PRO and RED membranes are in different stages of development. For example, the semi-permeable membranes in PRO are widely available while access to the ion exchange membranes in RED is still low. In addition, currently available IEMs were made specifically for electrodialysis (ED) making commercially developed and optimized RED membranes rare. ED converts salt water to fresh water and RED utilizes fresh water and salt water to create energy. The RED process is thus inversely related to ED. RED consists of alternating sets of cation exchange membranes (CEMs) and anion exchange membranes (AEMs) as seen in Figure 1.
In 1954, Pattle first developed the principal of RED[2] by proving that through the mixing of river and seawater, an energy source could be created. Weinstein et al. created a mathematical model to show the viability of RED power generation by focusing on the need for manufactured RED IEMs and better optimization of operating conditions for RED technology to advance at the macro-scale level of energy conversion.[5] Lacey concluded in 1980 that for RED cells to have maximum net output voltages, their membranes needed to have high selectivity and low electrical resistance.[6] Lacey further stated that to lower RED costs, membranes have to be durable, strong, and stable. Jagur-Grodzinski et al. studied the use of flow control to increase power in spacer patterns.[7] Turek and Bandura found in 2007 that membranes with a smaller ionic flow path, a shorter length and bigger width, were more effective in energy production at the industrial level.[8] In 2014, Daniilidis et al. also highlighted the need for affordable membranes with better power performance for RED to have commercial success.[9] IEMs are currently 2–3 times more expensive than the membranes used in reverse osmosis (RO) desalination processes.[1,10] Considering the fact that RO membranes have noticeably decreased in cost due to increased use of RO in water desalination, cost of RED IEMs may also decrease with higher global demand. For more stable power generation, advances in IEM development for RED are needed.
Some new studies focusing on RED IEM design have arisen aiming to showcase the potential of IEM development for energy production.[11~14] More specifically, these studies look at how RED performance is influenced by the electrochemical and physical properties of IEMs. However, more analyses of physical and electrochemical properties are needed despite these studies’ findings regarding membrane performance-determining properties in RED stacks. Also, to improve power density generation, more variety in the number of membranes prepared with different materials and methods is needed. With more research into permselectivity, resistance, and ion exchange capacity, more variety in membrane preparation is expected.
With the development and optimization of RED membranes becoming more commonplace in this research field, an in-depth look at IEMs is needed. One topic being an analysis detailing the different membrane materials, properties, preparation methods, and other parts found in an RED stack. Other focuses include a review of different RED IEM materials and the current most advanced membranes used in RED. A final topic detailing the main properties, physical and electrochemical, of ideal RED membranes needed for system performance will be addressed through the utilization of experimentally determined results.
2. Theoretical and Experimental Background
2.1 Reverse electrodialysis
A RED stack contains a series of anion exchange membranes (AEMs) and cation exchange membranes (CEMs), placed in an alternating pattern (Figure 1). When the salt water and freshwater are fed to the respective water compartments in contact with the membranes, the salt concentration difference between the two adjacent compartments drives ion migration: cations toward the cathode by permeating the CEMs, and anions in the opposite direction toward the anode. These ionic charge transfer converts to electron current via redox reactions at the electrodes by means of proper electrode rinse solution: oxidation at anode and reduction at cathode. Thus, the redox process is determined by the electrodes and electrode rinse used in the system. A system with the opposite electrode redox reaction and electrode rinse recirculation will lead to no net chemical reaction.
The theoretical salinity gradient power generation in an RED system with many cells has long been reported based on Kirchhoff’s law and based on the fact that the potential generated from different cells is additive.[5,15] The stack of N membrane pairs generates an open-circuit potential of Vstack (Volt), which can be calculated as:
| (1) |
where N is the number of stacks, R is the gas constant (J/mol/K), T is the absolute temperature (K), F is the Faraday constant (C/mol), α is the average apparent permselectivity of the membranes (%) in the stack, ac is the activity (mol/L) of the concentrated salt solution, and ad is the activity (mol/L) of the diluted salt solution.
According to Kirchhoff’s law, the power output (Watts) of the RED stacks is estimated as:
| (2) |
The power output is a function of overall stack resistance Rstack (Ω) and external load resistance RL (Ω), so the output power, W (Watts), is maximized when Rstack and RL are equal,[16,17] expressed as:
| (3) |
If the power density is defined as power output per unit membrane area, the maximum power density, Pmax (W/m2) is then calculated as:
| (4) |
where, A is the membrane area (m2).
2.2 RED membrane properties
There are many properties of IEM that affect its performance and application in RED system. The physicochemical characteristics that determine ion transport are of great importance in many applications. Ion transport of IEMs is characterized by permselectivity, ion exchange capacity (IEC), membrane resistance, and swelling degree. Such characteristics reflect the distribution of fixed charge groups in the membrane structure. Thus, the type of the fixed ion, its concentration, and its special distribution within the membrane are the most important factors to study membrane properties.[17] Considering the aim of RED is to generate electrical energy, the requirement for RED IEMs are more focused on ionic resistance/conductivity and permselectivity than other properties.[14,18]
The permselectivity and electrical resistance of the membrane are important properties of IEM, because it is directly related to the power output in RED.[19] The membrane resistance is measured experimentally and depicts the energy loss of ion movement in the membrane. The determination of membrane resistance is dependent on conditions such as solution concentration, temperature, and concentration gradient across the membrane. The membrane resistance is often measured in compartment cells using aqueous NaCl (0.5M) solutions. Based on the total cell resistance composition (i.e., Rcell=Rsol+Rmem), the membrane resistance (Rmem) is determined by subtracting the resistance measured in the blank test (Rsol) from the resistance measured with the membrane under investigation (Rcell). At a system level, the RED stack resistance should consider all components in a series, including electrodes, electrolytes, diffusion boundary layers at the membrane surface, and membranes. However, the resistance caused by electrodes may be considered negligible if the stack is scaled up to more than 20 cells with only two electrodes.[20] Therefore, the RED stack resistance (Ω) of N number of cells can be expressed as:
| (5) |
where, Rohmic is the membrane resistance attributed to the ionic transport through the membranes, RΔC is the resistance attributed to the reduced electromotive forces as a consequence of the change in the concentration of the bulk solution, and RBL is the boundary layer resistance due to concentration polarization.
The permselectivity is a measure of the ability for the membrane to prevent co-ions and only allow counter-ions from passing through the membrane. If the complete co-ion exclusion is achieved from the ideal membrane, such IEM would have a permselectivity of one. The permselectivity decreases below the ideal value of 1 as the solution concentration increases, because a certain portion of co-ions may always contribute to the transport current, which suggested by Donnan’s theory.[21,22] The permselectivity is determined experimentally typically using two testing compartment cells, filled with two solutions of different concentration in each cell (e.g., 0.1M and 0.5M NaCl). The permselectivity can be calculated from the ratio of the measured membrane potential (ΔVmeasured) over the theoretical membrane potential (ΔVtheoretical) as shown below:
| (6) |
where, α is the membrane permselectivity (%), ΔVmeasured is the measured membrane potential (V) and ΔVtheoretical is the theoretical membrane potential. Note that thetheoretical membrane potential is the membranepotential for an ideal 100% permselective membrane, which was estimated to be 0.0379 V from the Nernstequation.[12] In theory,[5~7] the permselectivity directlyrelates to the generated electromotive force (membranepotential) under given activities of ions in the concentratedand diluted compartments according tothe equation (1).
The relationship between the permselectivity and the electrical resistance of the membrane is complexed owing to its mutual correlation of ionic species groups and structural variation. In RED, high permselectivity may not be necessarily required in comparison to low resistance requirement. Considering that the primary goal of RED system is to produce electricity, high permselectivity is not strict requirement for such application.[19] The requirement of membrane resistance is highly crucial for an RED application because the improvement of membrane conductivity is an indispensable process of power maximization. Also, membrane resistance is the key parameter that determines system efficiency. On the other hand, the permselectivity is considered as more important for deionization process such as ED in which the purity of the water products is of greater concern.[23] This allows the resistance to be sacrificed to some extent. Therefore, well-balance between permselectivity and resistance are often preferred in the development of IEMs for superb performance in RED salinity gradient power generation process.[24]
The ion-exchange capacity (IEC) represents the number of milliequivalent of fixed ionic charges per unit weight of a dry membrane (i.e., meq/g). The CEMs mostly incorporate sulfonic acid groups or carboxylic acid groups in the polymer matrix, while AEMs merge with ammonium groups within their structure. Thus, the IEMs are often classified dependent upon type and distribution of ion exchange groups that formulates structure of the membrane. The IEC of IEMs is determined experimentally according to the titration method using a strong acid or base of HCl for CEMs and NaOH for AEMs, respectively. The swelling degree is affected by such structural nature of the membrane. Since swelling degree indicates the amount of water taken up by the membrane, the structural formation of fixed charged group (i.e., IEC) directly regulates the phenomenon. As more water molecules are absorbed and kept hold by membrane body, ionic functional groups of polymer matrix are disrupted and density of fixed charge groups decreases. Also, as more ionic charge groups are present, more swelling occurs and thus lower resistances are expected. Therefore, IEC and swelling degree are crucial membrane properties that are closely correlated with each other. The fixed charge density (FCD) is determined by the ratio of IEC and swelling degree, and describes the overall effect of the swelling on IEC of an IEM.[19,25] If the IEC and water uptake of a membrane (swelling) are known, the FCD (meq/g H2O) can be expressed as in Eq. (7)[17,26,30]:
| (7) |
where, FCD is the fixed charge density, and wu is the water uptake.
The FCD can be useful indicator when IEC and water content do not vary together. High permselectivity normally occurs with increased IEC of IEMs. Also, the water content or swelling in the membrane phase is influenced by the size of the free volume and concentration gradient based on osmosis deswelling effect in the membrane phase. Therefore, the overall FCD decreases as the membrane loses the level of permselectivity with the same degree of IEC. Such effect of deswelling is more noticeable and easily exhibits in AEMs than in CEMs.[21,24,26]
3. RED membranes and power performance
3.1 Commercial ion exchange membranes
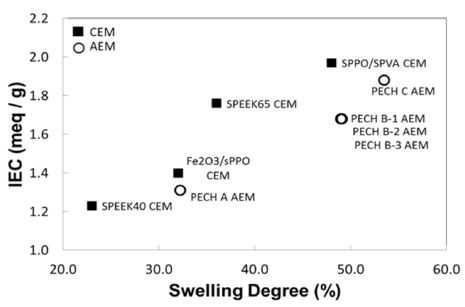
In this section, key properties of commercially available IEMs are analyzed in order to anticipate their power performance in RED system. The representative IEMs are selected based on their superb membrane performance and are comparatively analyzed based on their IEC, swelling degree, charge density, permselectivity and electrical resistance. For comparison, six commercially available CEMs and five commercially available AEMs are chosen for the analysis of key membrane properties and power performance under RED conditions: CEMs are Fumasep® FKD, FKS, Qianqiu, Neosepta® CMX, Selemion® CMV, and Ralex® CMH-PES. AEMs are Fumasep® FAD, FAS, Neosepta® AMX, Qianqiu, and Ralex® AMH-PES. Note that all IEMs are homogeneous membranes except for those two Ralex IEMs, which are heterogeneous. In Figure 2, the reported values of IEC and swelling degree are plotted. The IEC indicates the number of strong acids(-SO3-) groups in CEMs and strong basic (-NR3+) groups in the AEMs. However, these properties vary dependent upon the membranes owing to its different chemical composition and molecular structure. The highest IEC level is 2.34 meq/g dry exhibited with CMH-PES. Although high swelling is not desired, the membranes with high IEC often result in high swelling. The more ionic groups embedded with the membrane structure allows higher IEC, which also increases the hydrophilic nature of the membrane. Thus, the membrane swells more. Such trend is observed with selected IEMs especially with CEMs. In addition, the structure of the membrane affects the level of IEC and swelling. As seen in Figure 2, heterogeneous membranes (e.g., Ralex IEMs: CMH-PES and AMH-PES) show relatively higher IEC and swelling degree with possibly more amount of ion exchangeable species present within their structure than those other homogeneous membranes.
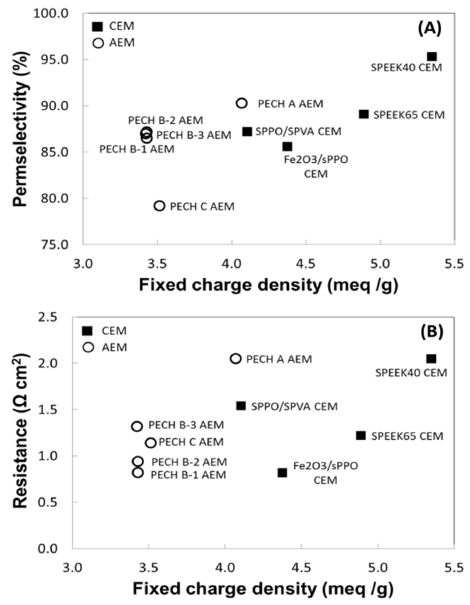
The FCD is an important electrochemical property, which indicates the number of charged ionic groups in the membrane. In Figure 3, we present the permselectivity and resistance of the commercial membranes as a function of FCD derived from IEC and swelling degree. As more charged groups are fixed, higher permselectivity and lower resistance are often expected. Despite their strong interconnectedness, the relationship between these properties is not always straightforward (Figure 3). The CEMs generally have higher permselectivity than AEMs at higher charge density. Only Fumasep CEM (i.e., FKD) is deviated from the general trend by having lower permselectivity than some AEMs. In Figure 3 (B), all the CEMs have a higher charge density, but their resistance levels are placed at a similar level as AEMs. Only heterogeneous Ralex CMH-PES seems to have a higher membrane resistance. This can be explained by increased mechanical strength from high degree of crosslinking, which also affects to increase the resistance. Also, such heterogeneous IEMs exhibit much higher resistance than homogeneous IEMs owing to their structure, which are not fully filled with charged groups in one polymer matrix.[21]
3.2 Custom-made ion exchange membranes
The importance of developing RED-specific membranes with low electrical resistance, high transport number, and high selectivity has been stressed in many studies.[11,18,19] The membranes used in RED are in an early stage of development. In addition, commercially developed and optimized membranes for RED application are rare primarily because the currently available IEMs are developed specifically for other applications such as ED. There have been some studies on designing custom-made IEMs for RED power generation, but it still remains challenging to tailor membranes featured with such desired properties. Also, the performance deterioration of membranes is an obstacle for commercialization, which requires further membrane performance optimization.
In this section, custom-made RED IEMs are evaluated. As seen in Table 1, four CEMs and six of fixed charge density for custom-made IEMs AEMs are selected for the analysis of key membrane properties and power performance under RED conditions: CEMs are Fe2O3/sPPO,[11,14] SPEEK 40, 65,[18] and SPPO/SPVA. AEMs are PECH A, B-1, B-2, B-3, C,[12] and PDDA/PVA. Note that two custom-made membranes, SPPO/SPVA and PDDA/PVA are selfdesigned and synthesized, thus the data reported in this review are obtained experimentally by author. A Figure 4 shows that SPPO/SPVA CEM and PECH C AEM have highest IEC: 2.0 and 1.88 meq/g dry respectively. Some custom-made membranes containing a high amount of active polymer have significantly higher swelling degrees, such as PECH C, which have a swelling degree of 53.5%. PECH C AEM with a relatively high IEC and swelling has also resulted lowest charge density of 3.5 meq/g H2O and permselectivity of 79.2% (Figure 5 (A)).
The membranes with high IEC often exhibit high swelling as well due to increased group of charged ionic species embedded in the membrane structure, which allow more water molecules absorbed in the polymer matrix. Hence, such high swelling also affects to disrupt the functional groups of polymer matrix, which was reflected in the result of FCD and permselectivity.
Since the FCD describes the quantity of counter ions that can be exchanged per unit mass of water in the membrane, higher FCD results the stronger co-ion exclusion.[27,28] Therefore, the selective transport of counter ions with increasing FCD will promote higher degree of permselectivity. Such trend of a high FCD that also improves permselectivity clearly shows with custom-made membranes as seen in Figure 5 (A). However, the impact of charge density on electrical resistance lacks the apparent relationship that exhibits random behavior of resistance with charge density (Figure 5 (B)). The resistance is the crucial property that directly determines the level of power performance in any electrochemical system, thus is an important considering factor in designing RED membranes. Similarly with the commercial membranes, the custom-made membranes did not show a linear trend between FCD and resistance, but the level of resistance on given range of FCD are noticeably lower than those of commercial membranes (Figure 5 (B)). This independent behavior of membrane resistance may results from increased nature of hydrophilicity on membrane surface with larger ionic species domain of polymer matrix or some inhomogeneous region of fixed charge distribution may also interfere smooth transport of counter ions through the membrane that lead the resistance to increase. Nonetheless, the lowest level of resistance is exhibited with PECH B AEMs and Fe2O3/sPPO CEM at 0.8-0.9 Ω cm2 (Figure 5 (B)).
3.3 Power performance in the RED system
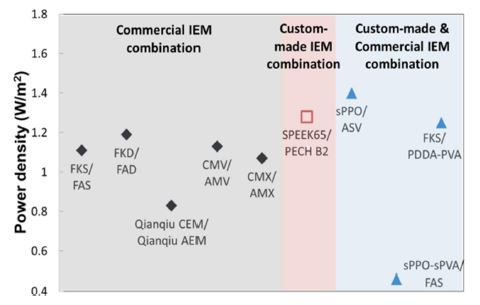
The power performance of different membrane pairs in RED stack is illustrated in Figure 6. The first group (shaded in grey) represents the experimental RED power performance from combined pair of commercial CEMs and AEMs, and the second group (shaded in pink) represents the pair solely from custom-made IEMs. Lastly, the group in blue shaded area represents the power results from the combination of custom-made and commercial IEMs. Since all the commercial membranes were produced to be used in ED applications, the membrane properties were not optimized specifically for RED. Our custom-made membranes on the other hand, had excellent electrochemical properties (e.g., electrical resistance) compared with those of commercial membranes, resulting in the higher experimental power output.
Figure 6 shows the highest power density reported from experimental data as 1.4 W/m2 using mix of custom-made CEM and commercial AEM pairs (i.e., Fe2O3/sPPO and ASV).[11] A comparison of electrochemical characteristics from previous section reveals that low resistance (0.8 Ω cm2) is the key property, which is enhanced by custom-made membrane. Such low resistance basically counteracts the negative effect of the less preferred, low permselectivity for the custom-made CEM Fe2O3/sPPO (i.e., 85.6%). A pair consisting of both custom-made membranes (SPEEK65 CEM / PECH B2 AEM), also resulted in good performance with relatively low resistance.[18] On the other hand, the permselectivity of those membranes did not seem crucial for achieving such high power density, which was placed at mediocre levels of 87.2-89.1%. By contrast, the performance of other commercial membranes is also hampered by such effects, which is reflected as nonideal behavior: commercial membrane pairs exhibited relatively lower power density with significantly high permselectivity and resistance. This implies that electrical resistance is the key parameter that majorly determines the power output.
4. Conclusions
For the salinity gradient power generation via RED process, the preparation of IEMs and understanding the properties and factors that determine the performance are the most crucial. This paper discussed the key physicochemical and electrochemical properties of the ideal RED membranes as well as important performance-determining RED phenomena using experimentally obtained characteristics. The urgency of membrane development and optimization for the RED power generation has been emphasized in many studies. A successful commercialization of salinity gradient energy depends on effective and cost-competitive membranes that are specially designed and carefully optimized to specific conditions for energy conversion technique such as RED process. With these advancements, the energy harvest from salinity gradients will become more practical and effective as one of the future renewable and sustainable energy sources of power.
Nomenclature
| RED : | reverse electrodialysis |
| ED : | electrodialysis |
| PRO : | pressured retarded osmosis |
| IEM : | ion exchange membrane |
| CEM : | cation exchange membranes |
| AEM : | anion exchange membranes |
| IEC : | ion exchange capacity |
| FCD : | fixed charge density |
| N : | number of stacks |
| R : | gas constant, J/mol/ K |
| T : | absolute temperature, K |
| F : | faraday constant, C/mol |
| α : | average apparent permselectivity |
| a : | activity of solution |
Subscript
| stack : | membrane stack |
| c : | concentrated salt solution |
| d : | diluted salt solution |
References
-
B.E. Logan, M. Elimelech, Membrane-based processes for sustainable power generation using water, Nature, 488, (2012), p313-319.
[https://doi.org/10.1038/nature11477]

-
R. Pattle, Production of electric power by mixing fresh and salt water in the hydroelectric pile, Nature, 174, (1954), p660.
[https://doi.org/10.1038/174660a0]

-
G.Z. Ramon, B.J. Feinberg, E.M.V. Hoek, Membrane-based production of salinity-gradient power, Energy & Environmental Science, 4, (2011), p4423.
[https://doi.org/10.1039/c1ee01913a]

-
J.W. Post, J. Veerman, H.V.M. Hamelers, G.J.W. Euverink, S.J. Metz, K. Nymeijer, C.J.N. Buisman, Salinity-gradient power: Evaluation of pressure-retarded osmosis and reverse electrodialysis, J Membrane Sci, 288, (2007), p218-230.
[https://doi.org/10.1016/j.memsci.2006.11.018]

-
J.N. Weinstein, F.B. Leitz, Electric power from differences in salinity: the dialytic battery, Science, 191, (1976), p557.
[https://doi.org/10.1126/science.191.4227.557]

-
R. Lacey, Energy by reverse electrodialysis, Ocean engineering, 7, (1980), p1-47.
[https://doi.org/10.1016/0029-8018(80)90030-X]

-
J. Jagur-Grodzinski, R. Kramer, Novel process for direct conversion of free energy of mixing into electric power, Industrial & Engineering Chemistry Process Design and Development, 25, (1986), p443-449.
[https://doi.org/10.1021/i200033a016]

-
M. Turek, B. Bandura, Renewable energy by reverse electrodialysis, Desalination, 205, (2007), p67-74.
[https://doi.org/10.1016/j.desal.2006.04.041]

-
A. Daniilidis, R. Herber, D.A. Vermaas, Upscale potential and financial feasibility of a reverse electrodialysis power plant, Applied Energy, 119, (2014), p257-265.
[https://doi.org/10.1016/j.apenergy.2013.12.066]

-
M. Elimelech, W.A. Phillip, The future of seawater desalination: energy, technology, and the environment, Science, 333, (2011), p712-717.
[https://doi.org/10.1126/science.1200488]

-
J.G. Hong, Y. Chen, Nanocomposite reverse electrodialysis (RED) ion-exchange membranes for salinity gradient power generation, J Membrane Sci, 460, (2014), p139-147.
[https://doi.org/10.1016/j.memsci.2014.02.027]

-
E. Guler, Y.L. Zhang, M. Saakes, K. Nijmeijer, Tailor-made anion-exchange membranes for salinity gradient power generation using reverse electrodialysis, Chemsuschem, 5, (2012), p2262-2270.
[https://doi.org/10.1002/cssc.201200298]

-
E. Guler, R. Elizen, M. Saakes, K. Nijmeijer, Micro-structured membranes for electricity generation by reverse electrodialysis, J Membrane Sci, 458, (2014), p136-148.
[https://doi.org/10.1016/j.memsci.2014.01.060]

-
J.G. Hong, Y. Chen, Evaluation of electrochemical properties and reverse electrodialysis performance for porous cation exchange membranes with sulfatefunctionalized iron oxide, J Membrane Sci, 473, (2015), p210-217.
[https://doi.org/10.1016/j.memsci.2014.09.012]

-
D.A. Vermaas, M. Saakes, K. Nijmeijer, Doubled power density from salinity gradients at reduced inter-membrane distance, Environmental science & technology, 45, (2011), p7089-7095.
[https://doi.org/10.1021/es2012758]

-
V. Shaposhnik, K. Kesore, An early history of electrodialysis with permselective membranes, J Membrane Sci, 136, (1997), p35-39.
[https://doi.org/10.1016/S0376-7388(97)00149-X]

-
R.K. Nagarale, G.S. Gohil, V.K. Shahi, Recent developments on ion-exchange membranes and electro-membrane processes, Advances in Colloid and Interface Science, 119, (2006), p97-130.
[https://doi.org/10.1016/j.cis.2005.09.005]

-
E. Guler, R. Elizen, D. Vermaas, M. Saakes, K. Nijmeijer, Performance-determining membrane properties in reverse electrodialysis, J Membrane Sci, (2013).
[https://doi.org/10.1016/j.memsci.2013.06.045]

-
P. Dlugolecki, K. Nymeijer, S. Metz, M. Wessling, Current status of ion exchange membranes for power generation from salinity gradients, J Membrane Sci, 319, (2008), p214-222.
[https://doi.org/10.1016/j.memsci.2008.03.037]

-
J. Veerman, M. Saakes, S. Metz, G. Harmsen, Reverse electrodialysis: evaluation of suitable electrode systems, Journal of Applied Electrochemistry, 40, (2010), p1461-1474.
[https://doi.org/10.1007/s10800-010-0124-8]

- H. Strathmann, Ion-exchange membrane separation processes, Elsevier Science, (2004).
- F.G. Helfferich, Ion exchange, McGraw-Hill, New York, (1962).
-
A. Grabowski, G.Q. Zhang, H. Strathmann, G. Eigenberger, The production of high purity water by continuous electrodeionization with bipolar membranes: Influence of the anion-exchange membrane permselectivity, Journal of Membrane Science, 281, (2006), p297-306.
[https://doi.org/10.1016/j.memsci.2006.03.044]

-
G.M. Geise, M.A. Hickner, B.E. Logan, Ionic resistance and permselectivity tradeoffs in anion exchange membranes, ACS applied materials & interfaces, 5, (2013), p10294-10301.
[https://doi.org/10.1021/am403207w]

-
A.H. Galama, J.W. Post, M.A.C. Stuart, P.M. Biesheuvel, Validity of the Boltzmann equation to describe Donnan equilibrium at the membrane-solution interface, Journal of Membrane Science, 442, (2013), p131-139.
[https://doi.org/10.1016/j.memsci.2013.04.022]

-
A.R. Khare, N.A. Peppas, Swelling/deswelling of anionic copolymer gels, Biomaterials, 16, (1995), p559-567.
[https://doi.org/10.1016/0142-9612(95)91130-Q]

-
A.V. Sokirko, J.A. Manzanares, J. Pellicer, The permselectivity of membrane systems with an inhomogeneous distribution of fixed charge groups, Journal of colloid and interface science, 168, (1994), p32-39.
[https://doi.org/10.1006/jcis.1994.1390]

-
Y. Mizutani, R. Yamane, H. Ihara, H. Motomura, Studies of ion exchange membranes. XVI. The preparation of ion exchange membranes by the “paste method”, Bulletin of the Chemical Society of Japan, 36, (1963), p361-366.
[https://doi.org/10.1246/bcsj.36.361]